West Chester-based Verrica Pharmaceuticals, a dermatology therapeutics company developing medications for skin diseases, recently re-submitted the new drug application for its VP-102 drug to the U.S. Food and Drug Administration (FDA).
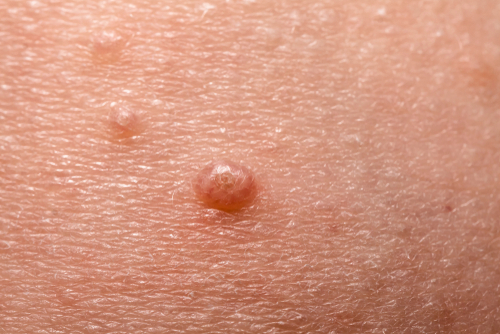
VP-102 is for the treatment of molluscum contagiosum (molluscum), a highly contagious viral skin disease. It is caused by a pox virus and produces raised, skin-toned-to-pink lesions that can cause pain, inflammation, itching, and bacterial infection.
There are no FDA-approved treatments for molluscum, and the rash lasts an average of 13 months but can last up to several years. In the United States, it affects approximately six million people, primarily children.
VP-102 is delivered via a single-use applicator that allows for precise topical dosing and targeted administration. Verrica intends to seek conditional approval to market VP-102 in the United States under the brand name YCANTH.
“We believe the successful tech transfer of our bulk solution manufacturing addresses the only deficiency in our previous filing that resulted in our complete response letter last year,” said Ted White, Verrica Pharmaceuticals president and CEO. “We look forward to working with the FDA through the review process and, if approved, bringing VP-102 to patients as the first FDA-approved treatment option for molluscum.”